Quick Details
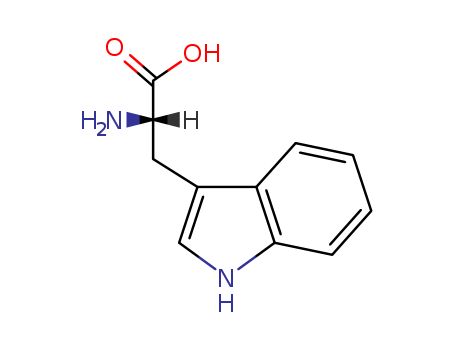
- ProName: L-Tryptophan
- CasNo: 73-22-3
- Molecular Formula: C11H12N2O2
- Appearance: White or yellow-white crystal or cryst...
- PackAge: 25kg/drum
- Purity: 99%
- Storage: 24 months
- LimitNum: 5 Kilogram
Superiority
Synonym: (S)-2-Amino-3-(3-indolyl)propionic acid
Molecular formula: C11H1N2O2
Molecular weight: 204.23
Appearance:
- White or yellow-white…
Details
Synonym: (S)-2-Amino-3-(3-indolyl)propionic acid
Molecular formula: C11H1N2O2
Molecular weight: 204.23
Appearance:
- White or yellow-white crystal or crystalline powder.
- Odorless of slight odor.
- Slight bitter taste.
- Slightly soluble in water
What is L-Tryptophan?
Tryptophan (IUPAC-IUBMB abbreviation: Trp or W; IUPAC abbreviation: L-Trp or D-Trp; sold for medical use as Tryptan) is one of the 20 standard amino acids, as well as an essential amino acid in the human diet. It is encoded in the standard genetic code as the codon UGG. Only the L-stereoisomer of tryptophan is used in structural or enzyme proteins, but the D-stereoisomer is occasionally found in naturally produced peptides (for example, the marine venom peptide contryphan). The distinguishing structural characteristic of tryptophan is that it contains an indole functional group. It is an essential amino acid as demonstrated by its growth effects on rats.
Where will L-Tryptophan be used for?
For many organisms (including humans), tryptophan is an essential amino acid. This means that it cannot be synthesized by the organism and therefore must be part of its diet. Amino acids, including tryptophan, act as building blocks in protein biosynthesis. In addition, tryptophan functions as a biochemical precursor for the following compounds (see also figure to the right):
?Serotonin (a neurotransmitter), synthesized via tryptophan hydroxylase.Serotonin, in turn, can be converted to melatonin (a neurohormone), via N-acetyltransferase and 5-hydroxyindole-O-methyltransferase activities.
?Niacin is synthesized from tryptophan via kynurenine and quinolinic acids as key biosynthetic intermediates.
?Auxin (a phytohormone) when sieve tube elements undergo apoptosis tryptophan is converted to auxins.
The disorders fructose malabsorption and lactose intolerance cause improper absorption of tryptophan in the intestine, reduced levels of tryptophan in the blood and depression.
In bacteria that synthesize tryptophan, high cellular levels of this amino acid activate a repressor protein, which binds to the trp operon. Binding of this repressor to the tryptophan operon prevents transcription of downstream DNA that codes for the enzymes involved in the biosynthesis of tryptophan. So high levels of tryptophan prevent tryptophan synthesis through a negative feedback loop and, when the cell's tryptophan levels are reduced, transcription from the trp operon resumes. The genetic organisation of the trp operon thus permits tightly regulated and rapid responses to changes in the cell's internal and external tryptophan levels.